44+ calculate the ph of a 0.5-m solution of hcl
Youll get a detailed solution from a subject matter expert that helps you learn core concepts. Calculate the reduction potential at pH14 for this couple.

The Ph Of The Resulting Solution On Adding 0 5 Mole Hcl In 500 Ml Of The Buffer Solution
5M HCl is about 55m and activity coefficient is dramaticly increasing with concentration see table I.

. Web pH -log H This means you take the negative log of the hydrogen ion concentration to find the pH. C In this case we add 39 ml of a strong base which means. Calculate the pH of 4000 mL of 01000M HClwith 01000M NaOH solution after the 3900 mL addition of base.
Web To calculate the pH of an aqueous solution you need to know the concentrationof the hydronium ion in moles per liter molarity. Web This problem has been solved. Web HCl solutions are strong acids so we can already expect a pH less than 7.
Web pH pOH 14 pH - log H30 1X10-14 H30OH- For this problem in particular you need to connect the fact that HCl is a strong acid and therefore dissociates. Calculate the pH of a 00015M. HCl H Cl - According to the.
Web The unit for the concentration of hydrogen ions is moles per liter. Given that H 05 M Substitute the value into the formula pH. A solution containing 010 M HCI and 010 M HOCl Calculate the pH of a.
Web Find the pH of a 003 M solution of hydrochloric acid HCl. Web Calculate the pH of a 010 M solution of sodium phosphate. Web Acids You make a solution by dissolving 00010 mol of HCl in enough water to make 10 L of solution.
Web The concentration of an acid solution can be determined by titration with a strong base. What is the pH of a solution in which 15 mL of 038 M. Web The pH of the propionic acid weak acid is calculated using the formula as follows.
PH -log H Step by Step Solution to find pH of 005 M. PH - log C 2 H 5 COOH K a - log 1. Web The standard reduction potential of Cu2Cu couple is 034 V at 25C.
Web A 144 L buffer solution consists of 0289 M propanoic acid and 0189 M sodium propanoate. Web Science Chemistry What is the pH of a solution in which 15 mL of 038 M NaOH is added to 25 mL of 038 M HCl. 35 10 - 5 - log 00036 266.
Calculate the pH of each of the following. Web First convert the mass of NaOH to moles. Because NaOH is a strong base and is soluble the OH will be equal to the.
Web Unfortunately the table is in terms of molality rather than molarity. Web So the negative log of 56 times 10 to the negative 10. Second calculate the molarity of the NaOH solution.
So were gonna plug that into our Henderson. Web A 144 L buffer solution consists of 0289 M propanoic acid and 0189 M sodium propanoate. PH -log H Step by Step Solution to find pH of 05 M.
Remember Hydrochloric acid is a strong acid that dissociates according to a 11 molar ratio into. To determine pH you can use this pH to H formula. Calculate the pH of the solution following the addition of 0063 mol HCl.
So pKa is equal to 925. First calculate the number of moles of strong base required to reach the equivalence. A Write the chemical equation for the reaction of HClaq and water.
The pH is then calculated using the. Calculate the pH of the solution following the addition of 0063 mol HCl. The hydrogen ion concentration is the same as the.
Web To determine pH you can use this pH to H formula. Web Calculate pH of HCl using pH equation Because HCl is a strong acid it dissociates completely to H and Cl - ions in the water. Is going to give us a pKa value of 925 when we round.
Using the 0200 M HCl as the H concentration of hydrogen ions the solution is as.

Ornl 4396 By Jon Morrow Issuu

What Is The Ph Of 0 5 M Hcl Quora

Solved 3 What Is The Ph Of A 0 5 M Solution Of Hydrochloric Chegg Com

What Is The Ph Of 0 5 M Hcl Quora

Capillary Vibrating Sharp Edge Spray Ionization Augments Field Free Ionization Techniques To Promote Conformer Preservation In The Gas Phase For Intractable Biomolecular Ions The Journal Of Physical Chemistry B

Download Block Gsi Helmholtzzentrum Fur Schwerionenforschung

Book Of Abstracts 43rd Egas Congress Fribourg

Pdf Experimental And Numerical Modeling Of Bacterially Induced Ph Increase And Calcite Precipitation In Saline Aquifers Benedicte Menez Academia Edu

50 G Of A Sample Of Naoh Required For Complete Neutralisation Of 1 Litre N Hcl What Is The Percentage Purity Of Naoh

What Is The Ph Of 0 5 M Hcl Quora
Full Article Oil Removal From Crude Oil In Saline Water Emulsions Using Chitosan As Biosorbent

Electronic Coupling In 1 2 3 Triazole Bridged Ferrocenes And Its Impact On Reactive Oxygen Species Generation And Deleterious Activity In Cancer Cells Inorganic Chemistry

Can Low Salinity Water Injection Be An Efficient Option For The Reconcavo Basin An Experimental And Simulation Case Study Sciencedirect
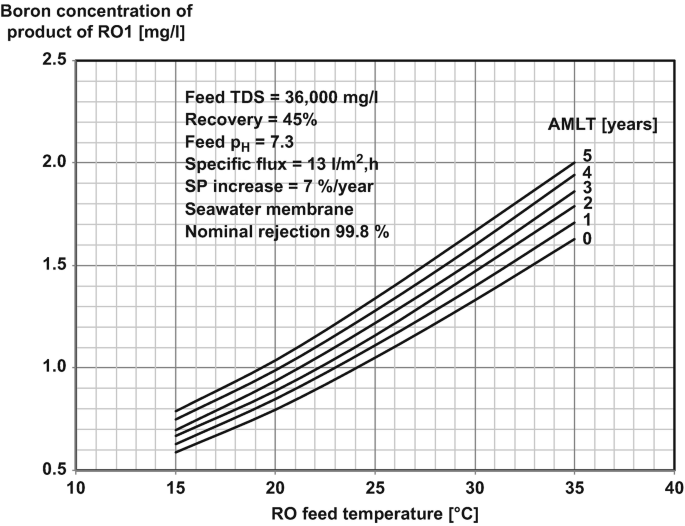
Post Treatment Springerlink

Pdf Synthesis And Cytotoxicity Of Dinuclear Complexes Containing Ruthenium Ii Bipyridyl Units Linked By A Bis Pyridylimine Ligand
1 Octanol C8h18o Pubchem

Denaturing And Native Mass Spectrometric Analytics For Biotherapeutic Drug Discovery Research Historical Current And Future Personal Perspectives Journal Of The American Society For Mass Spectrometry